Is Brcl5 Polar Or Nonpolar
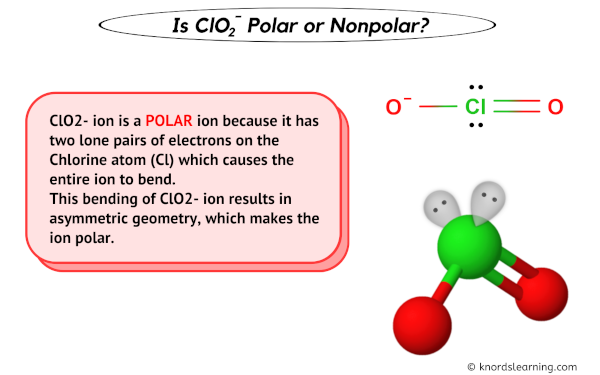
ClO2- (Chlorite ion) is a POLAR ion.
But why?
And how can you lot say that ClO2- ion is a polar ion?
Want to know the reason?
Allow's dive into it!
ClO2- ion is a POLAR ion because it has two alone pairs of electrons on the Chlorine atom (Cl) which causes the entire ion to bend.
This bending of ClO2- ion results in disproportionate geometry, which makes the ion polar.
Let me explicate this in particular with the assist of ClO2- lewis construction and its 3D geometry.
Why is ClO2- ion a Polar ion ? (Explained in 2 Steps)
To understand the polar nature of ClO2- ion, first of all you lot should know its lewis construction likewise as its molecular geometry.
So let's see this in the steps below.
Footstep #1: Draw the lewis structure
Hither is a skeleton of ClO2- lewis structure and information technology contains ii Chlorine-Oxygen bonds.
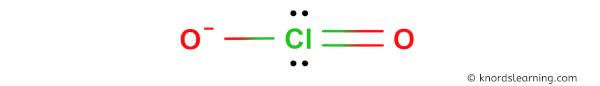
(Note: If you want to know the steps of drawing the ClO2- lewis dot construction, then visit this article: ClO2- lewis structure).
Then from the above diagram we take come to know that the ClO2- ion has two Chlorine-Oxygen bonds.
Now in the next step we have to check whether these two Chlorine-Oxygen bonds are polar or nonpolar.
And we as well have to cheque the molecular geometry of ClO2- ion.
Step #2: Check the bail polarity and molecular geometry
The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (ΔEN) between the 2 atoms.
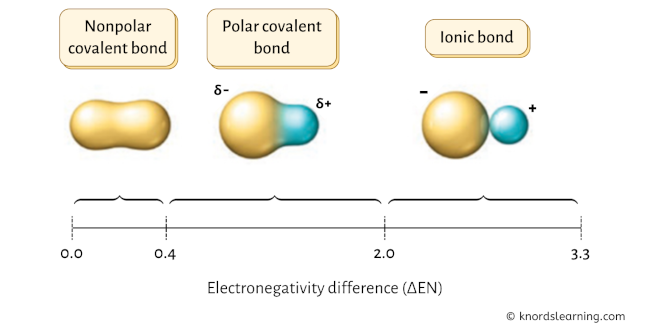
Have a await at the above paradigm.
- If the electronegativity difference ( ΔEN ) is less than 0.4, then the bond is nonpolar covalent bond.
- If the electronegativity deviation ( ΔEN ) is between 0.iv to two.0, then the bond is polar covalent bond.
- If the electronegativity difference ( ΔEN ) is greater than 2.0, so the bond is an ionic bond.
At present let's come to the instance of ClO2- ion. Information technology has ii Chlorine-Oxygen bonds.
You can come across the electronegativity values of Chlorine (Cl) and Oxygen (O) atoms from the periodic table given below.
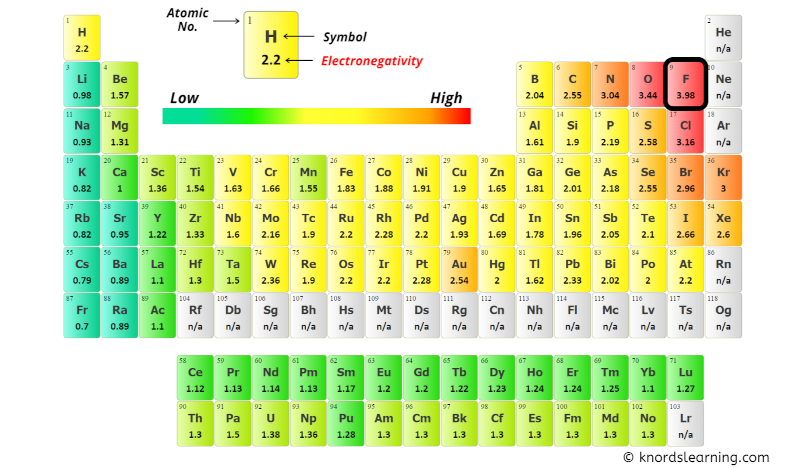
From the in a higher place image;
- Electronegativity of Chlorine (Cl) = 3.16
- Electronegativity of Oxygen (O) = 3.44
Now let'south meet the polarity of each bail.
For Chlorine-Oxygen bond;
The electronegativity divergence ( ΔEN ) = iii.44 – 3.sixteen = 0.28
This value is less than 0.4, which indicates that the bond between Chlorine (Cl) and Oxygen (O) is nonpolar.
Hence, each Chlorine-Oxygen bond is a nonpolar covalent bond.
But wait, nosotros as well take to look at the molecular geometry of ClO2- ion to know whether it has a symmetric shape or not.
Have a look at this 3D structure of ClO2- ion. The Chlorine atom (Cl) is at the center and it is surrounded by 2 Oxygen atoms (O).
It also has 2 alone pairs on the Chlorine atom (Cl).
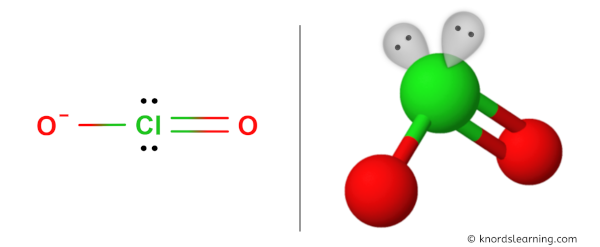
Due to the lone pair on the chlorine atom (Cl), its molecular geometry becomes asymmetric.
Because of this, there are positive and negative poles of charges on the overall ClO2- ion.
Hence, the ClO2- ion is a polar ion.
I promise you have understood the reason behind the polar nature of ClO2- ion.
See the polarity of other molecules to brand your concepts articulate:
Is XeCl4 Polar or Nonpolar?
Is NOBr Polar or Nonpolar?
Is GeH4 Polar or Nonpolar?
Is Isopropanol (C3H8O) Polar or Nonpolar?
Is Butane (C4H10) Polar or Nonpolar?
Is Brcl5 Polar Or Nonpolar,
Source: https://knordslearning.com/is-clo2-polar-or-nonpolar/
Posted by: minterolank1963.blogspot.com


0 Response to "Is Brcl5 Polar Or Nonpolar"
Post a Comment